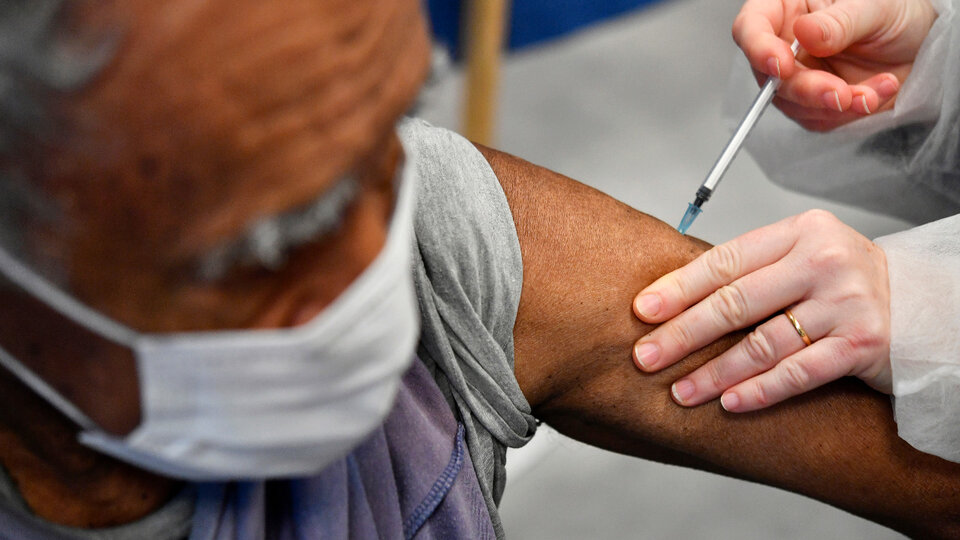
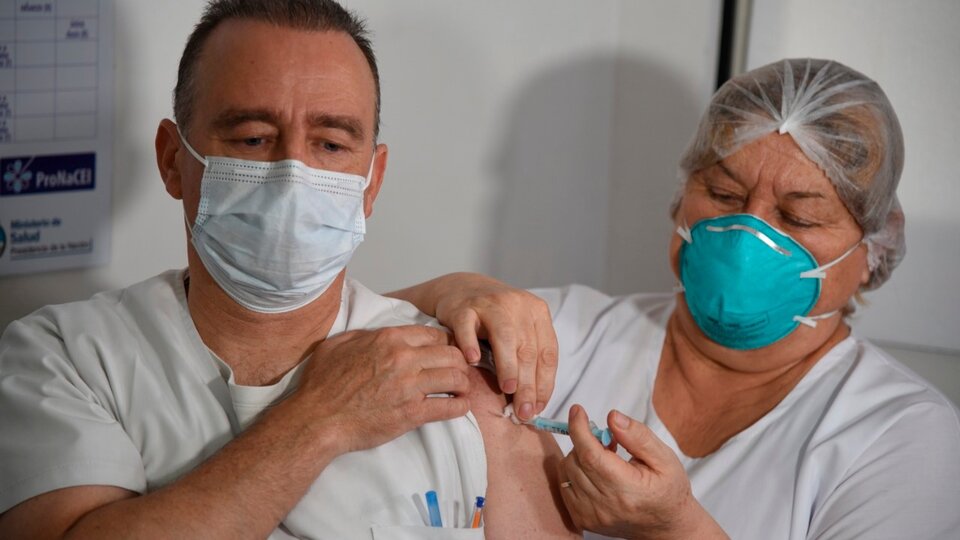
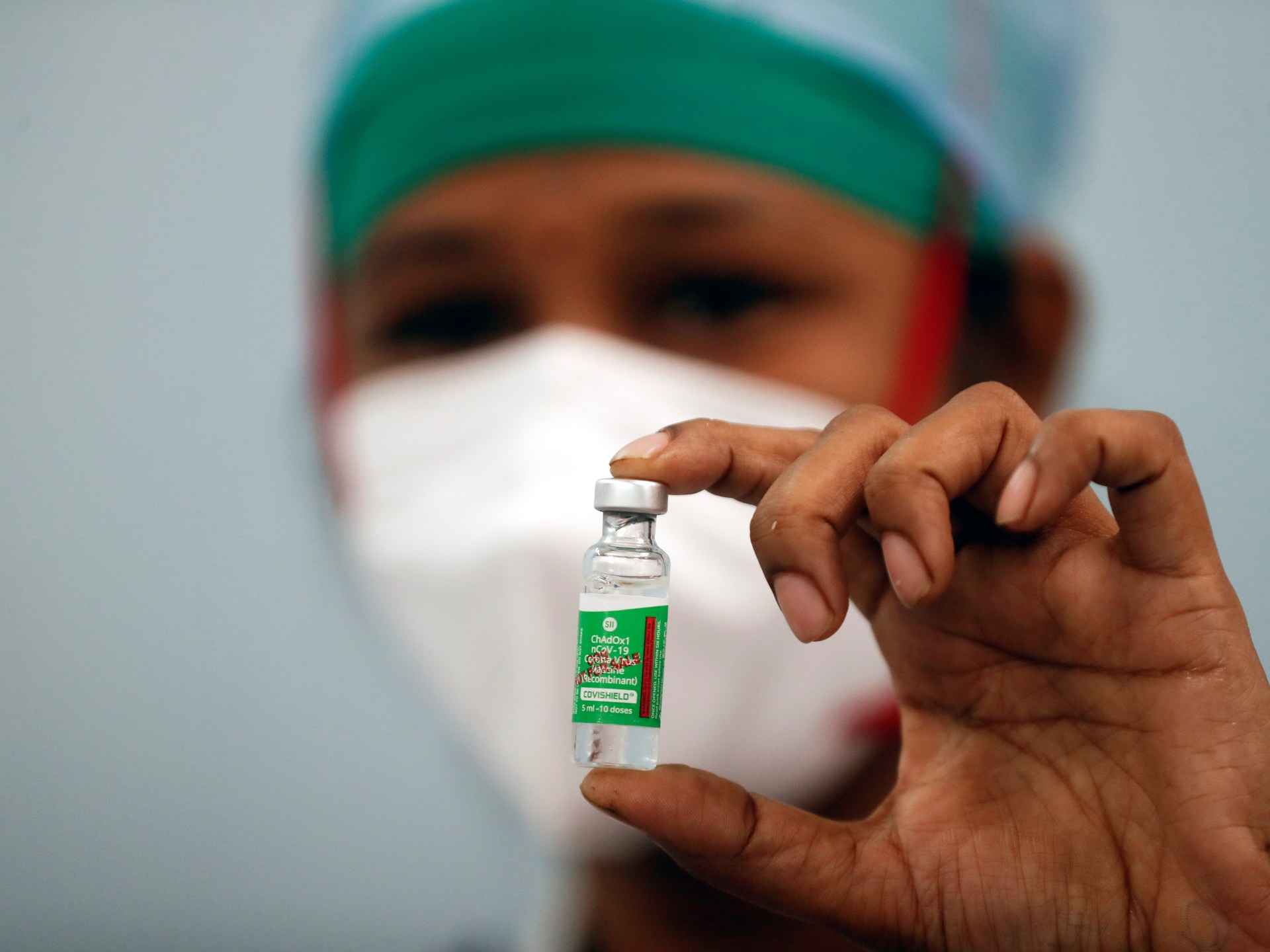
El ministro de Salud de la Nación, Ginés González García, supervisó hoy en el Hospital Posadas la aplicación de la segunda dosis de la vacuna Sputnik V contra la COVID-19 en el personal de salud que recibió la primera hace 21 días, plazo mínimo que debe haber entre dosis.

 www.argentina.gob.ar
“We are accessing the vaccines according to the contracts we have signed. We have more than 51 million doses insured with a national vaccination program throughout the country for when the campaign goes to scale. ” Ginés González García
www.argentina.gob.ar
“We are accessing the vaccines according to the contracts we have signed. We have more than 51 million doses insured with a national vaccination program throughout the country for when the campaign goes to scale. ” Ginés González García

El ministro supervisó la aplicación de la segunda dosis de la vacuna Sputnik V en el Hospital Posadas
Hoy las primeras personas que recibieron la primera dosis contra el virus SARS-CoV-2 cumplen el plazo mínimo para completar el esquema de vacunación con el segundo componente.
Last edited: