gracielle
Registered
- Joined
- Jun 6, 2005
- Messages
- 3,754
- Likes
- 3,100
Updated Oct. 27, 2021
29 October 2021
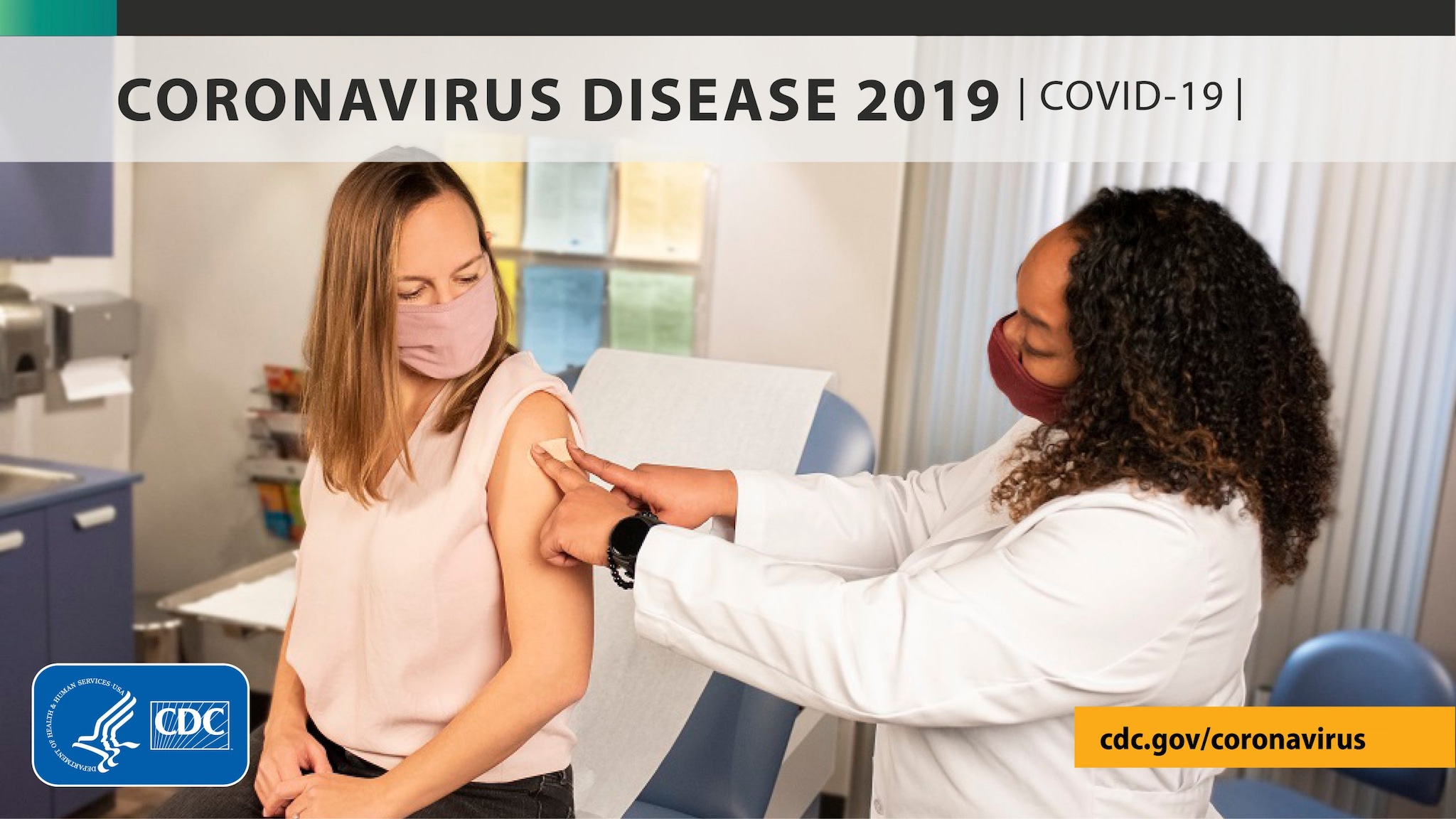
New CDC Study: Vaccination Offers Higher Protection than Previous COVID-19 Infection


29 October 2021
New CDC Study: Vaccination Offers Higher Protection than Previous COVID-19 Infection